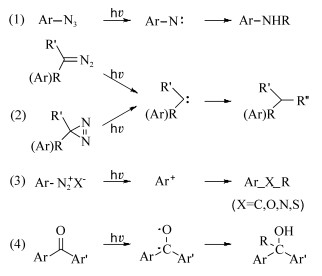
| [1] |
刑其毅, 徐瑞秋, 裴伟伟, 等.基础有机化学(第三版, 下册)[M].北京:高等教育出版社, 2005:712-746.XING Q Y, WU R Q, PEI W W, et al.. Basic Organic Chemistry[M]. Beijing:Higher Education Press, 2005:712-746.(in Chinese)
|
| [2] |
SINGH A, THORNTON E R, WESTHEIMER F H. The photolysis of diazoactylchymotrypsin[J]. Journal of Biological Chemistry, 1962, 237(9):3006-3008.
|
| [3] |
SUMRANJIT J, CHUNG S J. Recent advances in target characterization and identification by photoaffinity probes[J]. Molecules, 2013, 18(9):10425-40451. doi: 10.3390/molecules180910425
|
| [4] |
XIA Y, PENG L. Photoactivatable lipid probes for studying biomembranes by photoaffinity labeling[J]. Chemical Reviews, 2013, 113(10):7880-7929. doi: 10.1021/cr300419p
|
| [5] |
HATANAKA Y. Development and leading-edge application of innovative photoaffinity labeling[J]. Chemical & Pharmaceutical Bulletin, 2015, 46(1):1-12.
|
| [6] |
DAS J. Aliphatic diazirines as photoaffinity probes for proteins:recent developments[J]. Chemical Reviews, 2011, 111(8):4405-4417. doi: 10.1021/cr1002722
|
| [7] |
ITO Y. Photoimmobilization for microarrays[J]. Biotechnology Progress, 2006, 22(4):924-932. doi: 10.1021/bp060143a
|
| [8] |
ZHAO C W, ZHANG Z D, YANG W T. A remote photochemical reaction for surface modification of polymeric substrate[J]. Journal of Polymer Science Part A-polymer Chemistry, 2012, 50(18):3698-3702. doi: 10.1002/pola.v50.18
|
| [9] |
LAWRENCE E J, WILDGOOSE G G, ALDOUS L, et al.. 3-aryl-3-(trifluoromethyl)diazirines as versatile photoactivated "linker" molecules for the improved covalent modification of graphitic and carbon nanotube surfaces[J]. Chemistry of Materials, 2011, 23(16):3740-3751. doi: 10.1021/cm201461w
|
| [10] |
孟想, 杨蕊竹, 刘东旭, 等.紫外固化型聚合物水凝胶的周期图案形成及其调控[J].中国光学, 2012, 5(4):436-443. http://www.chineseoptics.net.cn/CN/abstract/abstract8772.shtmlMENG X, YANG R ZH, LIU D X, et al.. Formation and adjustment of cycle pattern of UV-curable polymeric hydeogel[J]. Chinese Optics, 2012, 5(4):436-443.(in Chinese) http://www.chineseoptics.net.cn/CN/abstract/abstract8772.shtml
|
| [11] |
刘东旭, 夏虹, 孙允陆, 等.飞秒激光直写生物凝胶模板原位合成纳米粒子[J].中国光学, 2014, 7(4):608-615. http://www.chineseoptics.net.cn/CN/abstract/abstract9150.shtmlLIU D X, XIA H, SUN Y L, et al.. Femtosecond laser direct writing bio-gel template for in situ synthesis of nanoparticles[J]. Chinese Optics, 2014, 7(4):608-615.(in Chinese) http://www.chineseoptics.net.cn/CN/abstract/abstract9150.shtml
|
| [12] |
FLEMING S A. Chemical reagents in photoaffinity labeling[J]. Tetrahedron, 1995, 51(46):12479-12520. doi: 10.1016/0040-4020(95)00598-3
|
| [13] |
BORDEN W T, GRITSAN N P, HADAD C M, et al.. The interplay of theory and experiment in the study of phenylnitrene[J]. Accounts of Chemical Research, 2000, 33(11):765-771. doi: 10.1021/ar990030a
|
| [14] |
PLATZ M S. Comparison of phenylcarbene and phenyinitrene[J]. Accounts of Chemical Research, 1995, 28(12):487-492. doi: 10.1021/ar00060a004
|
| [15] |
BLENCOWE A, HAYES W. Development and application of diazirine in biological and synthetic macromolecular systems[J]. Soft Matter, 2005, 1(3):178-205. doi: 10.1039/b501989c
|
| [16] |
AMBROZ H B, KEMP T J. Aryl cation-new light on old intermediates[J]. Chemical Society Reviews, 1979, 8(3):353-365. doi: 10.1039/cs9790800353
|
| [17] |
KOTZYBA-HIBERT F, KAPFER I, GOELDNER M. Recent trends in photoaffinity labeling[J]. Angewandte Chemie International Edition, 1995, 34(12):1296-1312. doi: 10.1002/(ISSN)1521-3773
|
| [18] |
DORMAN G, PRESTWICH G D. Benzophenone photophores in biochemistry[J]. Biochemistry, 1994, 33(19):5661-5673. doi: 10.1021/bi00185a001
|
| [19] |
DORMAN G, PRESTWICH G D, ELLIOTT J T, et al.. Benzophenone photoprobes for phosphoinositides, peptides and drugs[J]. Photochem Photobiol, 1997, 65(22):222-234.
|
| [20] |
AGARWAL S, BELL C M, ROTHBART S B, et al.. AMP-activated Protein Kinase(AMPK) Control of mTORC1 Is p53-and TSC2-independent in pemetrexed-treated carcinoma cells[J]. Journal of Biological Chemistry, 2015, 290(46):27473-27486. doi: 10.1074/jbc.M115.665133
|
| [21] |
BRODIE N I, MAKEPEACE K A T, PETROTCHENKO E V, et al.. Isotopically-coded short-range hetero-bifunctional photo-reactive crosslinkers for studying protein structure[J]. Journal of Proteomics, 2015, 118:12-20. doi: 10.1016/j.jprot.2014.08.012
|
| [22] |
YABE T, HOSODA-YABE R, SAKAI H, et al.. Development of a photoreactive probe-based system for detecting heparin[J]. Analytical Biochemistry, 2015, 472:1-6. doi: 10.1016/j.ab.2014.11.007
|
| [23] |
GUO H J, LI Z Q. Developments of bioorthogonal handle-containing photo-crosslinkers for photoaffinity labeling[J]. Med. Chem. Commun., 2017, 8(8):1585-1591. doi: 10.1039/C7MD00217C
|
| [24] |
FUNG S K, ZOU T T, CAO B, et al.. Cyclometalated gold(Ⅲ) complexes containing N-Heterocyclic carbene ligands engage multiple anti-cancer molecular targets[J]. Angewandte Chemie International Edition, 2017, 56(14):3892-3896. doi: 10.1002/anie.201612583
|
| [25] |
SCHWANSTECHER M, LÖSER S, CHUDZIAK F, et al.. Identification of a 38-kDa high affinity sulfonylurea-binding peptide in insulin-secreting cells and cerebral cortex[J]. Journal of Biological Chemistry, 1994, 269(27):17768-17771. http://cn.bing.com/academic/profile?id=cf1479bf74421fb56fa1e457e02a6b59&encoded=0&v=paper_preview&mkt=zh-cn
|
| [26] |
FRICK W, BAUERSCH FER A, BAUER J, et al.. Synthesis of a biotin-tagged photoaffinity probe of 2-azetidinone cholesterol absorption inhibitors[J]. Bioorganic & Medicinal Chemistry, 2003, 11(8):1639-1642. http://cn.bing.com/academic/profile?id=6952531eeab909814cc395b1535c79ac&encoded=0&v=paper_preview&mkt=zh-cn
|
| [27] |
ROTH M, CHEN W Y. Sorting out functions of sirtuins in cancer[J]. Oncogene, 2014, 33(13):1609-1620. doi: 10.1038/onc.2013.120
|
| [28] |
SEIFERT T, MALO M, LENGQVIST J, et al.. Identification of the binding site of chroman-4-one-based sirtuin 2-selective inhibitors using photoaffinity labeling in combination with tandem mass spectrometry[J]. Journal of Medicinal Chemistry, 2016, 59(23):10794-10799. doi: 10.1021/acs.jmedchem.6b01117
|
| [29] |
LIU K, SHI H B, XIAO H G, et al.. Functional profiling, identification and inhibition of plasmepsins in intraerythrocytic malaria parasites[J]. Angewandte Chemie International Edition, 2009, 48(44):8293-8297. doi: 10.1002/anie.200903747
|
| [30] |
SHI H B, LIU K, XU A, et al.. Small molecule microarray-facilitated screening of affinity-based probes(AfBPs) for γ-secretase[J]. Chemical Communications, 2009, 33(33):5030-5032. http://cn.bing.com/academic/profile?id=a3de8353cfadaba8314c03dd01b9bdf5&encoded=0&v=paper_preview&mkt=zh-cn
|
| [31] |
SHI H B, ZHANG C J, CHEN G Y, et al.. Cell-based proteome profiling of potential dasatinib targets by use of affinity-based probes[J]. Journal of the American Chemical Society, 2012, 134(6):3001-3014. doi: 10.1021/ja208518u
|
| [32] |
SHI H B, CHENG X M, YAO S Q, et al.. Proteome profiling reveals potential cellular targets of staurosporine using a clickable cell-permeable probe[J]. Chemical Communications, 2011, 47(40):11306-11308. doi: 10.1039/c1cc14824a
|
| [33] |
LI Z Q, HAO P L, LI L, et al.. Design and synthesis of minimalist terminal alkyne-containing diazirine photo-crosslinkers and their incorporation into kinase inhibitors for cell-and tissue-based proteome profiling[J]. Angewandte Chemie International Edition, 2013, 52(33):8551-8556. doi: 10.1002/anie.201300683
|
| [34] |
LI Z Q, WANG D Y, LI L, et al.. "Minimalist" cyclopropene-containing photo-cross-linkers suitable for live-cell imaging and affinity-based protein labeling[J]. Journal of the American Chemical Society, 2014, 136(28):9990-9998. doi: 10.1021/ja502780z
|
| [35] |
LI Z Q, QIAN L H, YAO S Q, et al.. Tetrazole photoclick chemistry:reinvestigating its suitability as a bioorthogonal reaction and potential applications[J]. Angewandte Chemie International Edition, 2016, 55(6):2002-2006. doi: 10.1002/anie.201508104
|
| [36] |
YU S H, BOYCE M, WANDS A M, et al.. Metabolic labeling enables selective photocrosslinking of O-GlcNAc-modified proteins to their binding partners[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(13):4834-4839. doi: 10.1073/pnas.1114356109
|
| [37] |
KRISHNAMURTHY M, DUGAN A, NWOKOYE A, et al.. Caught in the act:covalent crosslinking captures activator-coactivator interactions in vivo[J]. ACS Chemical Biology, 2016, 6(12):1321-1326. http://cn.bing.com/academic/profile?id=31b2f23a05790eebfbae77e5e0ad513b&encoded=0&v=paper_preview&mkt=zh-cn
|
| [38] |
SONG C X, HE C. Bioorthogonal labeling of 5-hydroxymethylcytosine in genomic DNA and diazirine-based DNA photo-cross-linking probes[J]. Accounts of Chemical Research, 2011, 44(9):709-717. doi: 10.1021/ar2000502
|
| [39] |
AND Y T, KOHLER J J. Photoactivatable crosslinking sugars for capturing glycoprotein interactions[J]. Journal of the American Chemical Society, 2008, 130(11):3278-3279. doi: 10.1021/ja7109772
|
| [40] |
BOND M R, WHITMAN C M, KOHLER J J. Metabolically incorporated photocrosslinking sialic acid covalently captures a ganglioside-protein complex[J]. Molecular Biosystems, 2010, 6(10):1796-1799. doi: 10.1039/c0mb00069h
|
| [41] |
YU D, WOWOR A J, COLE J L, et al.. Defining the Escherichia coli SecA dimer interface residues through in vivo site-specific photo-cross-linking[J]. Journal of Bacteriology, 2013, 195(12):2817-2825. doi: 10.1128/JB.02269-12
|
| [42] |
ZHANG M, LIN S, SONG X, et al.. A genetically incorporated crosslinker reveals chaperone cooperation in acid resistance[J]. Nature Chemical Biology, 2011, 7(10):671-677. doi: 10.1038/nchembio.644
|
| [43] |
LIN S, HE D, LONG T, et al.. Genetically encoded cleavable protein photo-cross-linker[J]. Journal of the American Chemical Society, 2014, 136(34):11860-11863. doi: 10.1021/ja504371w
|
| [44] |
YANG Y, SONG H P, HE D, et al.. Genetically encoded protein photocrosslinker with a transferable mass spectrometry-identifiable label[J]. Nature Communications, 2016, DOI: 10.1038/ncomms12299.
|
| [45] |
YAN H, ZHONG G, X U G, et al.. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus[J]. Elife, 2012, 1(1):e00049-e00049. http://cn.bing.com/academic/profile?id=305aba0d2c29d37474fb444fc26914bc&encoded=0&v=paper_preview&mkt=zh-cn
|
| [46] |
SHIGDEL U K, ZHANG J L, HE C. Diazirine-based DNA photo-cross-linking probes for the study of protein DNA interactions[J]. Angewandte Chemie International Edition, 2008, 47(1):90-93. doi: 10.1002/(ISSN)1521-3773
|
| [47] |
QIU Z H, LU L H, JIAN X, HE C. A diazirine-based nucleoside analogue for efficient DNA interstrand photocross-linking[J]. Journal of the American Chemical Society, 2008, 130(44):14398-14399. doi: 10.1021/ja805445j
|
| [48] |
NAKAMOTO K, UENO Y. Diazirine-containing RNA photo-cross-linking probes for capturing microRNA targets[J]. The Journal of Organic Chemistry, 2014, 79(6):2463-2472. doi: 10.1021/jo402738t
|
| [49] |
YAN M D, REN J. Covalent immobilization of ultrathin polymer films by thermal activation of perfluorophenyl azide[J]. Chemistry of Materials, 2004, 16(9):1627-1632. doi: 10.1021/cm034921v
|
| [50] |
LIU L, ENGELHARD M H, YAN M D. Surface and interface control on photochemically initiated immobilization[J]. Journal of the American Chemical Society, 2006, 128(43):14067-14072. doi: 10.1021/ja062802l
|
| [51] |
LIU L H, YAN M D. Functionalization of pristine graphene with perfluorophenyl azides[J]. Journal of Materials Chemistry, 2011, 21(10):3273-3276. doi: 10.1039/c0jm02765k
|
| [52] |
PARK J, JAYAWARDENA S N, CHEN X, et al.. A general method for the fabrication of grapheme-nanoparticle hybrid material[J]. Chemical Communications, 2015, 51(14):2882-2885. doi: 10.1039/C4CC07936A
|
| [53] |
ISMAILI H, LEE S, WORKENTIN M S. Diazirine-modified gold nanoparticle:template for efficient photoinduced interfacial carbene insertion reactions[J]. Langmuir, 2010, 26(18):14958-14964. doi: 10.1021/la102621h
|
| [54] |
ISMAILI H, LAGUGNE-LABARTHET F, WORKENTIN M S. Covalently assembled gold nanoparticle-carbon nanotube hybrids via a photoinitiated carbene addition reaction[J]. Chemistry of Materials, 2011, 23(6):1519-1525. doi: 10.1021/cm103284g
|
| [55] |
ISMAILI H, WORKENTIN M S. Covalent diamond gold nanojewel hybrids via photochemically generated carbenes[J]. Chemical Communications, 2011, 47(27):7788-7790. doi: 10.1039/c1cc12125a
|
| [56] |
GHIASSIAN S, ISMAILI H, LUBBOCK BRETT D W, et al.. Photoinduced carbene generation from diazirine modified task specific phosphonium salts to prepare robust hydrophobic coatings[J]. Langmuir, 2012, 28(33):12326-12333. doi: 10.1021/la301975u
|
| [57] |
GHIASSIAN S, BIESINGER M C, WORKENTIN M S. Synthesis of small water-soluble diazirine-functionalized gold nanoparticles and their photochemical modification[J]. Canadian Journal of Chemistry, 2015, 93(1):98-105. doi: 10.1139/cjc-2014-0287
|
| [58] |
SUN R, YIN L, ZHANG S H, et al.. Simple light-triggered fluorescent labeling of silica nanoparticles for cellular imaging applications[J]. Chemistry-A European Journal, 2017, 23(56):13893-13896. doi: 10.1002/chem.201703653
|
| [59] |
BAN Q F, BAI T, DUAN X, et al.. Noninvasive photothermal cancer therapy nanoplatforms via integrating nanomaterials and functional polymers[J]. Biomaterials Science, 2017, 5(2):190-210. doi: 10.1039/C6BM00600K
|
| [60] |
MIESZAWSKA A J, MULDER W J M, FAYAD Z A, et al.. Multifunctional gold nanoparticles for diagnosis and therapy of disease[J]. Molecular Pharmaceutics, 2013, 10(3):831-847. doi: 10.1021/mp3005885
|
| [61] |
李欣远, 纪穆为, 王虹智, 等.近红外光热转换纳米晶研究进展[J].中国光学, 2017, 10(5):541-554. http://www.chineseoptics.net.cn/CN/abstract/abstract9545.shtmlLI X Y, JI M W, WANG H ZH, et al.. Research progress of near-infrared photothermal conversion nanocrystals[J]. Chinese Optics, 2017, 10(5):541-554.(in Chinese) http://www.chineseoptics.net.cn/CN/abstract/abstract9545.shtml
|
| [62] |
CHENG X J, SUN R, YIN L, et al.. Light-triggered assembly of gold nanoparticles for photothermal therapy and photoacoustic imaging of tumors in vivo[J]. Advanced Materials, 2017, DOI: 10.1002/adma.201604894.
|
| [63] |
COYNE C P, JONES T, BEAR R. Synthesis of a covalent epirubicin-(C3-amide)-anti-HER2/neu immunochemotherapeutic utilizing a UV-photoactivated anthracycline intermediate[J]. Cancer Biotherapy and Radiopharmaceuticals, 2012, 27(1):41-55. doi: 10.1089/cbr.2011.1097
|
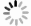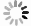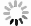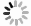





 下载:
下载: