Application of optical tweezers technology in physical chemistry characterization of aerosol
-
摘要: 有机气溶胶的热/动力学研究是多学科交叉的前沿研究领域,其核心问题主要是非理想混合包括挥发性、液-液相分离、非平衡传质动力学等,精确测量这些过程相关理化参数是目前研究的瓶颈。光镊系统可以悬浮气溶胶单颗粒,获得高信噪比受激拉曼光谱,在研究气溶胶物理化学性质与其大气效应中具有独到优势。被广泛用于有机及其与无机混合体系气溶胶的吸湿性、挥发性、水传质动力学、液-液相分离过程研究中。本文综述了激光悬浮气溶胶单颗粒技术的研究进展,主要包括光镊技术的原理和技术手段,以及在气溶胶关键物理化学参数测量中的应用。通过光镊系统,一方面可以获得重要理化参数的精确结果,另一方面可以对实际环境中悬浮液滴的状态进行模拟测量,从而为大气科学的研究与污染治理提供重要支撑。Abstract: Investigation of thermodynamics and kinetics process of organic aerosol is a cross-cutting field of multidisciplinary research, in which core issues are non-ideal mixing, including volatility, liquid-liquid phase separation and non-equilibrium mass transfer kinetics. At present, the study of the accurate measurement of the relevant physical and chemical parameters of these processes enters the bottleneck period. The optical tweezers system allows the aerosol single particles to be in a suspended state, resulting in a high signal-to-noise ratio of the stimulated Raman spectra. The system has unique advantages in the study of the physical and chemical properties of aerosols and its atmospheric effects. The system has been widely used in the research of the hygroscopicity, volatility, water mass transfer kinetics, and liquid-liquid phase separation processes of organic and inorganic mixture system aerosol. In this review, the progress of laser aerosol single particle technology is reviewed, including the principle and technical means of optical tweezers technology and the measurement of key physical and chemical parameters of aerosols. The results show that on one hand, accurate results of important physical and chemical parameters can be obtained by optical tweezers; on the other hand, the state of suspended droplets can be simulated and measured in the actual environment, which provides important support for atmospheric science research and pollution control.
-
Key words:
- optical tweezers /
- stimulated Raman spectra /
- aerosol
-
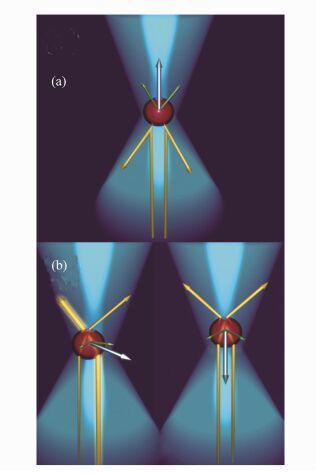
图 1 (a)散射力:反射的光束产生方向相反的动量构成了沿激光方向的合力,当颗粒不在光束的中心时,光束强度的变化会产生使颗粒向中间靠拢的合力;(b)梯度力:光束发生折射产生水平方向的合力,当颗粒在光束的中心时,光束产生的合力正对着颗粒[27-28]
Figure 1. (a)Scattering force: reflection of rays produces momentum in the opposite direction, resulting in a net force along the direction of laser propagation. (b)Gradient force:refraction of rays produces momentum in the opposite direction, resulting in a force vertical to the direction of laser propagation, when the bead is laterally centered in the beam, the net force points toward the focal point of the beam[27-28]
图 9 (a)模间距变化判定相分离发生的实例;(b)利用模消失现象判定相分离发生的实例;(c)利用拟合误差升高现象判定相分离发生的实例
Figure 9. (a)Illustration of LLPS with changed mode offset, corresponds to core shell structure; (b)Illustration of LLPS with quenching of mode, corresponds to partial engulfing structure; (c)Illustration of LLPS with increasing fitting error, corresponds to formation of complex state in the droplet
图 10 不同比例聚乙二醇/硫酸铵体系的相分离RH。灰色阴影表示Ciobanu等[60]的研究结果。前人研究中体相溶液实验,显微镜加湿实验和显微镜去湿实验的结果分别用红色、绿色和蓝色实心方块表示。对应模间距变化判据、模消失判和拟合误差上升判据的结果分别用红色、绿色和蓝色空心方块表示
Figure 10. Phase-separation relative humidities observed from the PEG:AS single-particle measurements. The shaded region is the two liquid phase-one-liquid phase boundary from the phase diagram presented by Ciobanu et al.[60] with the envelope indicating the range of uncertainty. Filled blue, green, and red squares indicate liquid-liquid phase separation RHs(SRHs) measured from microscope drying experiments, microscope wetting experiments, and bulk phase experiments, respectively. The red, green, and blue open squares show the averaged data from all phase separation RHs identified by CMO, QM, and IFE signatures, respectively. Error bars for this work show the range of SRHs obtained at each composition
图 12 不同RH下MgSO4液滴半径变化半衰期与RH变化半衰期比例。空心三角形表示红外实验结果,实心圆表示光镊实验结果。如图中的图例所示,(a)中不同颜色表示不同是半衰期比例,(b)中不同的颜色表示不同的RH变化量
Figure 12. Ratio between half-life of MgSO4 droplet radius stage and half-life of its corresponding RH stage. FTIR measurement results and optical tweezers measurement results are shown with opened triangles and solid circles, respectively. Mapped colours in (a) and (b) represents t1/2, radii/t1/2, RH and ΔRH, respectively
表 1 不同比例聚乙二醇/硫酸铵体系的相分离RH结果
Table 1. SRH results of PEG/AS system with different PEG:AS ratio
OIR 平均相分离RH 相分离RH范围 1:1 91.5 90.9~92.8 1:3 93.2 92.6~93.8 3:1 88.9 86.4 ~91.5 1:9 89.2 88.6~89.8 9:1 90.5 88.3~93.0 表 2 不同RH变化范围对应的体相水分扩散系数
Table 2. Diffusion coefficient of water in droplet bulk phase at different RH scales
过程 起始RH 最终RH 起始半径R0/μm Dap/10-16 m2/s 水分挥发过程 39.9 28.7 3.85 1.67±0.15 28.2 17.0 3.73 1.80±0.15 16.6 5.8 3.73 1.93±0.17 水分凝聚过程 29.9 40.2 3.84 6.42±0.50 17.7 29.1 3.73 1.61±0.13 4.6 16.7 3.71 0.97±0.08 -
[1] MAXWELL J C. Treatise on Electricity and Magnetism[M]. Oxford(UK):Clarendon Press, 1873. [2] LEBEDEV P N. Experimental examination of light pressure[J]. Annalen der Physik, 1901, 6:433. https://es.scribd.com/document/76312661/Experimental-Examination-of-Light-Pressure-Annalen-Der-Physik-Pyotr-Lebedev-1901-English [3] ASHKIN A, DZIEDZIC J M, BJORKHOLM J E, et al.. Observation of a single-beam gradient force optical trap for dielectric particles[J]. Optical Letter, 1986, 11(5):288-290. doi: 10.1364/OL.11.000288 [4] POSCHL U. Atmospheric aerosols:composition, transformation, climate and health effects[J]. Angewandte Chemie International Edition, 2005, 44(46):7520-7540. doi: 10.1002/(ISSN)1521-3773 [5] IPCC International Panel On Climate, Climate Change 2007-The Physical Science Basis:Working Group Ⅰ Contribution to the Fourth Assessment Report of the IPCC[M]. Cambridge(UK):Cambridge University Press, 2007. [6] JIMENEZ J L, CANAGARATNA M R, DONAHUE N M, et al.. Evolution of organic aerosols in the atmosphere[J]. Science, 2009, 326(5959):1525-1529. doi: 10.1126/science.1180353 [7] HALLQUIST M, WENGER J C, BALTENSPERGER U, et al.. The formation, properties and impact of secondary organic aerosol:current and emerging issues[J]. Atmospheric Chemistry and Physics, 2009, 9(14):5155-5236. doi: 10.5194/acp-9-5155-2009 [8] CHANG E I, PANKOW J F. Prediction of activity coefficients in liquid aerosol particles containing organic compounds, dissolved inorganic salts, and water-Part 2:consideration of phase separation effects by an X-UNIFAC model[J]. Atmospheric Environment, 2006, 40(33):6422-6436. doi: 10.1016/j.atmosenv.2006.04.031 [9] ZUEND A, SEINFELD J H. Modeling the gas-particle partitioning of secondary organic aerosol:the importance of liquid-liquid phase separation[J]. Atmospheric Chemistry and Physics, 2012, 12(9):3857-3882. doi: 10.5194/acp-12-3857-2012 [10] SONG M, MARCOLLI C, KRIEGER U K, et al.. Liquid-liquid phase separation in aerosol particles:dependence on O:C, organic functionalities, and compositional complexity[J]. Geophysical Research Letters, 2012, 39(19):L19801. http://authors.library.caltech.edu/35393/1/2012GL052807.pdf [11] POEHLKER C, WIEDEMANN K T, SINHA B, et al.. Biogenic potassium salt particles as seeds for secondary organic aerosol in the amazon[J]. Science, 2012, 337(6098):1075-1078. doi: 10.1126/science.1223264 [12] YOU Y, RENBAUM-WOLFF L, CARRERAS-SOSPEDRA M, et al.. Images reveal that atmospheric particles can undergo liquid-liquid phase separations[J]. Proceedings of the National Academy of Sciences U.S.A., 2012, 109(33):13188-13193. doi: 10.1073/pnas.1206414109 [13] ZOBRIST B, MARCOLLI C, PEDERNERA D A, et al.. Do atmospheric aerosols form glasses?[J]. Atmospheric Chemistry and Physics, 2008, 8(17):5221-5244. doi: 10.5194/acp-8-5221-2008 [14] MIKHAILOV E, VLASENKO S, MARTIN S T, et al.. Amorphous and crystalline aerosol particles interacting with water vapor:conceptual framework and experimental evidence for restructuring, phase transitions and kinetic limitations[J]. Atmospheric Chemistry and Physics, 2009, 9(24):9491-9522. doi: 10.5194/acp-9-9491-2009 [15] SHIRAIWA M, AMMANN M, KOOP T, et al.. Gas uptake and chemical aging of semisolid organic aerosol particles[J]. Proceedings of the National Academy of Sciences U.S.A., 2011, 108(27):11003-11008. doi: 10.1073/pnas.1103045108 [16] KOOP T, BOOKHOLD J, SHIRAIWA M, et al.. Glass transition and phase state of organic compounds:dependency on molecular properties and implications for secondary organic aerosols in the atmosphere[J]. Physical Chemistry Chemical Physics, 2011, 13(43):19238-19255. doi: 10.1039/c1cp22617g [17] VIRTANEN A, JOUTSENSAARI J, KOOP T, et al.. An amorphous solid state of biogenic secondary organic aerosol particles[J]. Nature, 2010, 467(7317):824-827. doi: 10.1038/nature09455 [18] SAUKKO E, LAMBE A T, MASSOLI P, et al.. Humidity-dependent phase state of SOA particles from biogenic and anthropogenic precursors[J]. Atmospheric Chemistry and Physics, 2012, 12(16):7517-7529. doi: 10.5194/acp-12-7517-2012 [19] CAPPA C D, WILSON K R. Evolution of organic aerosol mass spectra upon heating:implications for OA phase and partitioning behavior[J]. Atmospheric Chemistry and Physics, 2011, 11(5):1895-1911. doi: 10.5194/acp-11-1895-2011 [20] VADEN T D, IMRE D, BERANEK J, et al.. Evaporation kinetics and phase of laboratory and ambient secondary organic aerosol[J]. Proceedings of the National Academy of Sciences U.S.A., 2011, 108(6):2190-2195. doi: 10.1073/pnas.1013391108 [21] KALBERER M, PAULSEN D, SAX M, et al.. Identification of polymers as major components of atmospheric organic aerosols[J]. Science, 2004, 303(5664):1659-1662. doi: 10.1126/science.1092185 [22] KANAKIDOU M, SEINFELD J H, PANDIS S N, et al.. Organic aerosol and global climate modelling:a review[J]. Atmospheric Chemistry and Physics, 2005, 5:1053-1123. doi: 10.5194/acp-5-1053-2005 [23] MCFIGGANS G, TOPPING D O, BARLEY M H. The sensitivity of secondary organic aerosol component partitioning to the predictions of component properties-Part 1:A systematic evaluation of some available estimation techniques[J]. Atmospheric Chemistry and Physics, 2010, 10(21):10255-10272. doi: 10.5194/acp-10-10255-2010 [24] SHIRAIWA M, SEINFELD J H. Equilibration timescale of atmospheric secondary organic aerosol partitioning[J]. Geophysical Research Letters, 2012, 39(24):L24801. [25] PERRAUD V, BRUNS E A, EZELL M J, et al.. Nonequilibrium atmospheric secondary organic aerosol formation and growth[J]. Proceedings of the National Academy of Sciences U.S.A., 2012, 109(8):2836-2841. doi: 10.1073/pnas.1119909109 [26] HOPKINS R J, MITCHEM L, WARD A D, et al.. Control and characterisation of a single aerosol droplet in a single-beam gradient-force optical trap[J]. Physical Chemistry Chemical Physics, 2004, 6(21):603-625. [27] CHRISTINE P. Optical tweezers:not just for physicists anymore[J]. Analytical Chemistry, 2009, 81:16-19. doi: 10.1021/ac8023203 [28] WILLS J B, KNOX K J, REID J P. Optical control and characterization of aerosol[J]. Chemical Physics Letters, 2009, 481:153-165. doi: 10.1016/j.cplett.2009.09.020 [29] ASHKIN A, DZIEDZIC J M. Observation of optical resonances of dielectric spheres by light scattering[J]. Applied Optics, 1981, 20:1803-1814. doi: 10.1364/AO.20.001803 [30] BENNER R E, BARBER P W, OWEN J F, et al.. Observation of Structure Resonances in the Fluorescence Spectra from Microspheres[J]. Physical Review Letters, 1980, 44:475-478. doi: 10.1103/PhysRevLett.44.475 [31] BOHREN C F, HUFFMAN D R. Absorption and Scattering of Light by Small Particles[M]. New York:John Wiley and Sons, Inc., 1983. [32] HILL S C, BENNER R E. Morphology-Dependent Resonances[M]//CHANG R K, BARBER P W. Optical Effects Associated with Small Particles, World Scientific Publishing Co. Pte Ltd., 1988. [33] EVERSOLE J D, LIN H B, CAMPILLO A J. Input-out resonance correlation in laser-induced emission from micro droplets[J]. J. Optical Society of America B-Optical Physics, 1995, 12:287-296. doi: 10.1364/JOSAB.12.000287 [34] SYMES R, SAYER R M, REID J P. Cavity enhanced droplet spectroscopy:Principles, perspectives and prospects[J]. Physical Chemistry Chemical Physics, 2004, 6:474-487. doi: 10.1039/b313370b [35] BARTH H G. Modern Methods of Particle Size Analysis[M]. New York:John Willey & Sons, 1984:135-150. [36] MIE G. Beitrage zur Optik truber Medien, speziellkoloidaler Metallosungen[J]. Annalen der Physik, 1908, 25:377-445. [37] WRIEDT T. Mie theory 1908, on the mobile phone 2008[J]. J. Quantitative Spectroscopy and Radiative Transfer, 2008, 109:1543-1548. doi: 10.1016/j.jqsrt.2008.01.009 [38] PÖSCHL U. Atmospheric Aerosols:composition, transformation, climate and health effects[J]. Angewandte Chemie International Edition, 2005, 44:7520-7540. doi: 10.1002/(ISSN)1521-3773 [39] KANAKIDOU M, SEINFELD J H, PANDIS S N, et al.. Organic aerosol and global climate modeling:a review[J]. Atmospheric Chemistry and Physics, 2005, 5:1053-1123. doi: 10.5194/acp-5-1053-2005 [40] BILDE M, PANDIS S N. Evaporation rates and vapor pressures of individual aerosol species formed in the atmospheric oxidation of α and β-pinene[J]. Environmental Science & Technology, 2001, 35:3344-3349. doi: 10.1021/es001946b [41] CHANG E I, PANKOW J F. Prediction of activity coefficients in liquid aerosol particles containing organic compounds, dissolved inorganic salts, and water-part 2:consideration of phase separation effects by an X-UNIFAC model[J]. Atmospheric Environment, 2006, 40(33):6422-6436. doi: 10.1016/j.atmosenv.2006.04.031 [42] ZUEND A, SEINFELD J H. Modeling the gas-particle partitioning of secondary organic aerosol:the importance of liquid-liquid phase separation[J]. Atmospheric Chemistry and Physics, 2012, 12(9):3857-3882. doi: 10.5194/acp-12-3857-2012 [43] PANKOW J F. Gas/particle partitioning of neutral and ionizing compounds to single and multi-phase aerosol particles.1.Unified modeling framework[J]. Atmospheric Environment, 2003, 37(24):3323-3333. doi: 10.1016/S1352-2310(03)00346-7 [44] ZUEND A, SEINFELD J H. A practical method for the calculation of liquid-liquid equilibria in multicomponent organic-water-electrolyte systems using physicochemical constraints[J]. Fluid Phase Equlibria, 2013, 337:201-213. doi: 10.1016/j.fluid.2012.09.034 [45] ZUEND A, MARCOLLI C, LUO B P, et al.. A thermodynamic model of mixed organic-inorganic aerosols to predict activity coefficients[J]. Atmospheric Chemistry and Physics, 2008, 8(16):4559-4593. doi: 10.5194/acp-8-4559-2008 [46] ZUEND A, MARCOLLI C, BOOTH A M, et al.. New and extended parameterization of the thermodynamic model AIOMFAC:calculation of activity coefficients for organic-inorganic mixtures containing carboxyl, hydroxyl, carbonyl, ether, ester, alkenyl, alkyl, and aromatic functional groups[J]. Atmospheric Chemistry and Physics, 2011, 11(17):9155-9206. doi: 10.5194/acp-11-9155-2011 [47] JIMENEZ J L, CANAGARATNA M R, DONAHUE N M, et al.. Evolution of organic aerosols in the atmosphere[J]. Science, 2009, 326(5959):1525-1529. doi: 10.1126/science.1180353 [48] YOU Y, RENBAUM-WOLFF L, CARRERAS-SOSPEDRA M, et al.. Images reveal that atmospheric particles can undergo liquid-liquid phase separations[J]. Proceedings of the National Academy of Sciences U.S.A., 2012, 109(33):13188-13193. doi: 10.1073/pnas.1206414109 [49] BERTRAM A K, MARTIN S T, HANNA S J, et al.. Predicting the relative humidities of liquid-liquid phase separation, efflorescence, and deliquescence of mixed particles of ammonium sulfate, organic material, and water using the organic-to-sulfate mass ratio of the particle and the oxygen-to-carbon elemental ratio of the organic component[J]. Atmospheric Chemistry and Physics, 2011, 11(21):10995-11006. doi: 10.5194/acp-11-10995-2011 [50] REID J P, DENNIS-SMITHER B J, KWAMENA N-O A. The morphology of aerosol particles consisting of hydrophobic and hydrophilic phases:hydrocarbons, alcohols and fatty acids as the hydrophobic component[J]. Physical Chemistry Chemical Physics, 2011, 13(34):15559-15572. doi: 10.1039/c1cp21510h [51] SONG M, MARCOLLI C, KRIEGER U K, et al.. Liquid-liquid phase separation and morphology of internally mixed dicarboxylic acids/ammonium sulfate/water particles[J]. Atmospheric Chemistry and Physics, 2012, 12(5):2691-2712. doi: 10.5194/acp-12-2691-2012 [52] SHIRAIWA M, PFRANG C, KOOP T, et al.. Kinetic multi-layer model of gas-particle interactions in aerosols and clouds(KM-GAP):linking condensation, evaporation and chemical reactions of organics, oxidants and water[J]. Atmospheric Chemistry and Physics, 2012, 12(5):2777-2794. doi: 10.5194/acp-12-2777-2012 [53] SMITH M L, KUWATA M, MARTIN S T. Secondary organic material produced by the dark ozonolysis of alpha-pinene minimally affects the deliquescence and efflorescence of ammonium sulfate[J]. Aerospace Science and Technology, 2011, 45(2):244-261. doi: 10.1080/02786826.2010.532178 [54] MIKHAILOV E, VLASENKO S, MARTIN S T, et al.. Amorphous and crystalline aerosol particles interacting with water vapor:conceptual framework and experimental evidence for restructuring, phase transitions and kinetic limitations[J]. Atmospheric Chemistry and Physics, 2009, 9(24):9491-9522. doi: 10.5194/acp-9-9491-2009 [55] SHIRAIWA M, SEINFELD J H. Equilibration timescale of atmospheric secondary organic aerosol partitioning[J]. Geophysical Research Letters, 2012, 39(24):L24801. [56] ZUEND A, MARCOLLI C, LUO B P, et al.. A thermodynamic model of mixed organic-inorganic aerosols to predict activity coefficients[J]. Atmospheric Chemistry and Physics, 2008(8):4559. http://authors.library.caltech.edu/36057/1/acp-8-4559-2008.pdf [57] MITCHEM L, BUAJARERN J, WARD A D, et al.. A Strategy for characterizing the mixing state of immiscible aerosol components and the formation of multiphase aerosol particles through coagulation[J]. J. Physical Chemistry B, 2006, 110(28):13700-13703. doi: 10.1021/jp062874z [58] LAURAIN A M C, REID J P. Characterizing internally mixed insoluble organic inclusions in aqueous aerosol droplets and their influence on light absorption[J]. Journal of Physical Chemistry A, 2009, 113(25):7039-7047. doi: 10.1021/jp902248p [59] SONG M, MARCOLLI C, KRIEGER U K, et al.. Morphologies of mixed organic/inorganic/aqueous aerosol droplets[J]. Faraday Discussion, 2013, 165:289-316. doi: 10.1039/c3fd00049d [60] CIOBANU V G, MARCOLLI C, KRIEGER U K, et al.. Liquid-liquid phase separation in mixed organic/inorganic aerosol particles[J]. J. Physical Chemistry A, 2009, 113(41):10966-10978. doi: 10.1021/jp905054d [61] MARTIN S T. Transitions of aqueous atmospheric particles[J]. Chemical Review, 2000, 100:3403-3454. doi: 10.1021/cr990034t [62] ZOBRIST B, MARCOLLI C, PEDERNERA D A, et al.. Do atmospheric aerosols form glasses?[J]. Atmospheric Chemistry and Physics, 2008, 8:5221-5244. doi: 10.5194/acp-8-5221-2008 [63] HUFFMAN J A, DOCHERTY K S, AIKEN A C, et al.. Chemically-resolved aerosol volatility measurements from two megacity field studies[J]. Atmospheric Chemistry and Physics, 2009, 9:7161-7182. doi: 10.5194/acp-9-7161-2009 [64] POPE F P, DENNIS-SMITHER B J, GRIFFITHS P T, et al.. Studies of single aerosol particles containing malonic acid, glutaric acid, and their mixtures with sodium chloride. i. hygroscopic growth[J]. J. Physical Chemistry A, 2010, 114:5335-5341. doi: 10.1021/jp100059k?src=recsys [65] POPE F P, TONG H J, DENNIS-SMITHER B J, et al.. Studies of single aerosol particles containing malonic acid, glutaric acid, and their mixtures with sodium chloride.ii.liquid-state vapor pressures of the acids[J]. J. Physical Chemistry A, 2010, 114(37):10156-10165. doi: 10.1021/jp1052979 [66] ZIEGER P, FIERZ-SCHMIDHAUSER R, GYSEL M, et al.. Effects of relative humidity on aerosol light scattering in the Arctic[J]. Atmospheric Chemistry and Physics, 2010, 10:3875-3890. doi: 10.5194/acp-10-3875-2010 [67] HENZLER M, STOCK A, B L M. Adsorption on Ordered Surfaces on Ionic Solids and Thin Films[M]. Berlin:Springer, 1993. [68] TANG I N, MUNKELWITZ H R. Composition and temperature dependence of the deliquescence properties of hygroscopic aerosols[J]. Atmospheric Environment, 1993, 27A (4):467-473. [69] SHINDO H, OHASHI M, TATEISHI O, et al.. Atomic force microscopic observation of step movements on NaCl(001) and NaF(001) with the help of adsorbed water[J]. J. Chemical Society Faraday Transactions, 1997, 93(6):1169-1174. doi: 10.1039/a606256c [70] DAI Q, SALMERON M. Adsorption of water on NaCl (100) surfaces:role of atomic steps[J]. Journal of Physical Chemistry B, 1997, 101:1994-1998. doi: 10.1021/jp9625772 [71] WISE M E, MARTIN S T, RUSSELL L M, et al.. Water uptake by NaCl particles prior to deliquescence and the phase rule[J]. Aerospace Science and Technology, 2008, 42:281-294. doi: 10.1080/02786820802047115 [72] PÓSFAI M, BUSECK P R. Nature and climate effects of individual tropospheric aerosol particles[J]. Annual Review of Earth and Planetary Sciences, 2010, 38:17-43. doi: 10.1146/annurev.earth.031208.100032 [73] CHENLO F, MOREIRA R, PEREIRA G, et al.. Viscosities of aqueous solutions of sucrose and sodium chloride of interest in osmotic dehydration processes[J]. J. Food Engineering, 2002, 54:347-352. doi: 10.1016/S0260-8774(01)00221-7 [74] MCGRAW R, LEWIS E R. Deliquescence and efflorescence of small particles[J]. J. Chemical Physics, 2009, 131(19):194705. doi: 10.1063/1.3251056 [75] REINHARDT A, EMMENEGGER C, GERRITS B, et al.. Ultrahigh mass resolution and accurate mass measurements as a tool to characterize oligomers in secondary organic aerosols[J]. Analytical Chemistry, 2007, 79:4074-4082. doi: 10.1021/ac062425v [76] BADGER C L, GEORGE I, GRIFFITHS P T, et al.. Phase transitions and hygroscopic growth of aerosol particles containing humic acid and mixtures of humic acid and ammonium sulphate[J]. Atmospheric Chemistry and Physics, 2006, 6:755-768. doi: 10.5194/acp-6-755-2006 [77] CRUZ C N, PANDIS S N. Deliquescence and hygroscopic growth of mixed inorganic-organic atmospheric aerosol[J]. Environmental Science & Technology, 2000, 34:4313-4319. [78] KWAMENA N-O A, BUAJARERN J, REID J P. Equilibrium morphology of mixed organic/inorganic/aqueous aerosol droplets:investigating the effects of relative humidity and surfactants[J]. J. Physical Chemistry A, 2010, 114:5787-5795. doi: 10.1021/jp1003648 [79] MIKHAILOV E, VLASENKO S, MARTIN S T, et al.. Amorphous and crystalline aerosol particles interacting with water vapor:conceptual framework and experimental evidence for restructuring, phase transitions and kinetic limitations[J]. Atmospheric Chemistry and Physics, 2009, 9:9491-9522. doi: 10.5194/acp-9-9491-2009 [80] HARGREAVES G, KWAMENA N O, ZHANG Y-H, et al.. Measurements of the equilibrium size of supersaturated aqueous sodium chloride droplets at low relative humidity using aerosol optical tweezers and an electrodynamic balance[J]. J. Physical Chemistry A, 2010, 114(4):1806-1815. doi: 10.1021/jp9095985 [81] WALKER J S, WILLS J B, REID J P, et al.. Direct comparison of the hygroscopic properties of ammonium sulfate and sodium chloride aerosol at relative humidities approaching saturation[J]. J. Physical Chemistry A, 2010, 114(48):12682-12691. doi: 10.1021/jp107802y [82] SHIRAIWA M, ZUEND A, BERTRAM A K, et al.. Gas-particle partitioning of atmospheric aerosols: interplay of physical state, non-ideal mixing and morphology[J]. Physical Chemistry Chemical Physics, 2013, 15: 11441. doi: 10.1039/c3cp51595h -





 下载:
下载: