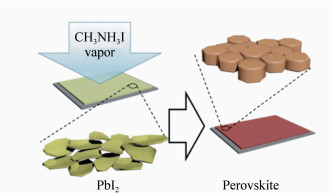
Preperation of perovskite materials and perovskite solar cells by vapor-assisted solution process
-
摘要: 有机无机杂化钙钛矿材料被广泛应用于光电器件领域,特别是其作为太阳能电池的吸光材料,受到学术界和工业界越来越多的关注。钙钛矿太阳能电池的产业化进程正在进行中,而在进一步降低制备成本、提高电池转换效率的同时,研究出一种操作简单且可重复性高的制备钙钛矿薄膜的技术具有十分重要的意义。与其他传统的溶液处理方法不同,蒸汽辅助溶液过程(VASP)处理法避免了薄膜在生长过程中溶解以及溶剂化作用,抑制了晶核的形成,使薄膜快速重组,获得致密的高质量钙钛矿薄膜。目前报道,基于此薄膜制备的平面结构钙钛矿太阳能电池转换效率高达16.8%。本文综述了低温(<150℃)VASP法制备的钙钛矿薄膜及光伏器件的相关研究进展,并对该技术的产业化前景做了展望。VASP制备过程简单、薄膜性能优异且可重复性高,为进一步制备大面积、高质量薄膜提供了可能。Abstract: Hybrid perovskites (e.g., CH3NH3PbX3, X represents a halide) are attracting more and more attention from both industry and academic due to their widely applications in the field of photoelectric device, especially as light absorption material in solar cells. The process of industrialization of perovskite solar cells is in progress, and in the pursuit of low-cost and efficient perovskite PV technology, it is crucial to develop a facile and high reproducible technique for preparing perovskite films. Unlike other conventional solution treatments, the vapor-assisted solution process(VASP) treatment avoids the dissolution and solvation of the film during growth, inhibits the formation of crystal nucleus and allows rapid recombination of the film to obtain dense high-quality perovskite film. At present, the conversion efficiency of planar structure perovskite solar cells based on this film is reaching up to 16.8%. In this paper, the research progress of perovskite thin films and photovoltaic devices prepared by low temperature( < 150℃) VASP method is reviewed. This paper also prospects the industrialization of the technology. VASP has the advantages of simple preparation process, excellent performance and high reproducibility, which provides the possibility of further preparation of large-area and high-quality film.
-
Key words:
- hybrid perovskite /
- vapor-assisted solution process /
- solar cell /
- photovoltaics
-
图 2 VASP法制备的钙钛矿薄膜[32]。(a)X射线衍射图谱[32]; (b)扫描电镜图谱[32]; (c)原子力显微镜图谱[32]; (d)截面扫描电镜图谱[32]; (e)吸收图谱[32]; (f)光致发光图谱[32]
Figure 2. Experiment perovskite film obtained via vapor-assisted solution process[32]:(a)X-ray diffraction pattern[32]; (b)top-view scanning electron microscopy image[32]; (c)tapping-mode atomic force microscopy height images[32]; (d)cross-sectional SEM image[32]; (e)absorption coefficient[32]; (f)photoluminescence[32]
图 3 VASP法制备的钙钛矿薄膜[32]。(a)薄膜分别退火0 h, 0.5 h, 4 h对应X射线衍射图谱[32];(b)退火0 h薄膜的扫描电镜图谱[32];(c)退火0.5 h薄膜的扫描电镜图谱[32];(d)退火4 h薄膜的扫描电镜图谱[32]
Figure 3. Experiment perovskite film obtained via vapor-assisted solution process[32] (a)X-ray diffraction patterns of the film annealed at 0, 0.5 and 4 h; (b)the film at initial stage at 0 h; (c)the film at the intermediate stage at 0.5 h; (d)the film at the post-stage at 4 h
图 5 (a)mCVT方法制备钙钛矿薄膜的XRD图谱[41]; (b)PbI2与CH3NH3I通过两步溶液旋涂法制备钙钛矿薄膜XRD图谱[41]; (c)PbCl2与CH3NH3I通过一步溶液旋涂法制备钙钛矿薄膜XRD图谱[41]
Figure 5. (a)XRD characterization of the perovskite films prepared by mCVT[41]. (b)Two step solution processing using PbI2 and CH3NH3I[41]. (c)One step processing using PbCl2 and CH3NH3I as reactants[41]. The samples are stored in air with 40%RH under darkness
-
[1] 章激扬, 李达, 杨苹, 等.光伏发电发展趋势分析[J].可再生能源, 2014, 32(2):127-132.ZHANG J Y, LI D, YANG P, et al.. Development trene analysis of photovoltaic power generation renewable energy resources, 2014, 32(2):127-132.(in Chinese) [2] LABORATORY N R E. Best Research-Cell Efficiencies; http://www.nrel.gov/ncpv/images/efficiency_chart.jpg. [3] GREEN M A, HO-BAILLIE A, SNAITH H J. The emergence of perovskite solar cells[J]. Nature Photonics, 2014, 8(7):506-514. doi: 10.1038/nphoton.2014.134 [4] SNAITH H J. Perovskites:the emergence of a new era for low-cost, high-efficiency solar cells[J]. The J. Physical Chemistry Letters, 2013, 4(21):3623-3630. doi: 10.1021/jz4020162 [5] PARK N-G. Perovskite solar cells:an emerging photovoltaic technology[J]. Materials Today, 2015, 18(2):65-72. doi: 10.1016/j.mattod.2014.07.007 [6] 苏彦勋, 柯沅锋, 蔡士良, 等.层层自组装金纳米粒子表面等离子体引发光电流应用于等离子体增感太阳能电池[J].中国光学, 2014, 7(2):267-273. http://www.chineseoptics.net.cn/CN/abstract/abstract9127.shtmlSU Y H, KE Y F, CAI SH L, et al.. Layer self-assembly of gold nanoparticles surface plasmon triggered photoelectric current applied plasmon sensitized solar cell[J]. Chinese Optics, 2014, 7(2):267-273.(in Chinese) http://www.chineseoptics.net.cn/CN/abstract/abstract9127.shtml [7] 蒋文程.环保型钙钛矿太阳能电池研制成功[J].中国光学, 2014, 7(4):682-683. http://www.cnki.com.cn/Article/CJFDTOTAL-NYYX201403023.htm [8] IM J H, LEE C R, LEE J W, et al.. 6.5% efficient perovskite quantum-dot-sensitized solar cell[J]. Nanoscale, 2011, 3(10):4088-4093. doi: 10.1039/c1nr10867k [9] KIM H S, LEE C R, IM J H, et al.. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%[J]. Scientific Reports, 2012, 2:591. doi: 10.1038/srep00591 [10] WEHRENFENNIG C, EPERON G E, JOHNSTON M B, et al.. High charge carrier mobilities and lifetimes in organolead trihalide perovskites[J]. Advanced Materials, 2014, 26(10):1584-1589. doi: 10.1002/adma.201305172 [11] XING G, MATHEWS N, SUN S, et al. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3[J]. Science, 2013, 342(6156):344. doi: 10.1126/science.1243167 [12] STRANKS S, EPERON G, GRANCINI G, et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber[J]., 2013, 342(6156):341-344. [13] DUAN H S, ZHOU H, CHEN Q, et al.. The identification and characterization of defect states in hybrid organic-inorganic perovskite photovoltaics[J]. Physical Chemistry Chemical Physics, 2014, 17(1):112-116. http://www.ncbi.nlm.nih.gov/pubmed/25354141 [14] JENG J-Y, CHIANG Y-F, LEE M-H, et al.. CH3NH3PbI3 perovskite/fullerene planar-heterojunction hybrid solar cells[J]. Advanced Materials, 2013, 25(27):3727-3732. doi: 10.1002/adma.v25.27 [15] LIU M, JOHNSTON M B, SNAITH H J. Efficient planar heterojunction perovskite solar cells by vapour deposition[J]. Nature, 2013, 501(7467):395-398. doi: 10.1038/nature12509 [16] LIU D, KELLY T L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques[J]. Nature Photonics, 2013, 8(2):133-138. doi: 10.1038/nphoton.2013.342 [17] MALINKIEWICZ O, YELLA A, LEE Y H, et al.. Perovskite solar cells employing organic charge-transport layers[J]. Nature Photonics, 2013, 8(2):128-132. doi: 10.1038/nphoton.2013.341 [18] YIN W J, SHI T, YAN Y. Unique properties of halide perovskites as possible origins of the superior solar cell performance[J]. Adv. Mater., 2014, 26(27):4653-4658. doi: 10.1002/adma.v26.27 [19] YIN W-J, SHI T, YAN Y. Superior photovoltaic propertiesof lead halide perovskites:insights from first-principles theory[J]. The J. Physical Chemistry C, 2015, 119(10):5253-5264. doi: 10.1021/jp512077m [20] 朱劲松, 李伟, 宋春花, 等. 元素替代对层状钙钛矿Bi4Ti3O12铁电薄膜的铁电性影响[C]. 中国光学学会2004年学术大会, 杭州, 2004: 72-76. [21] BURSCHKA J, PELLET N, MOON S J, et al.. Sequential deposition as a route to high-performance perovskite-sensitized solar cells[J]. Nature, 2013, 499(7458):316-319. doi: 10.1038/nature12340 [22] JEON N J, NOH J H, KIM Y C, et al.. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells[J]. Nat. Mater, 2014, 13(9):897-903. doi: 10.1038/nmat4014 [23] LEE J W, SEOL D J, CHO A N, et al.. High-efficiency perovskite solar cells based on the black polymorph of HC(NH2)2 PbI3[J]. Adv. Mater., 2014, 26(29):4991-4998. doi: 10.1002/adma.201401137 [24] WANG Q, SHAO Y, DONG Q, et al.. Large fill-factor bilayer iodine perovskite solar cells fabricated by a low-temperature solution-process[J]. Energy Environ. Sci., 2014, 7(7):2359-2365. doi: 10.1039/C4EE00233D [25] XIAO Z, BI C, SHAO Y, et al.. Efficient, high yield perovskite photovoltaic devices grown by interdiffusion of solution-processed precursor stacking layers[J]. Energy & Environmental Science, 2014, 7(8):2619. http://www.nrcresearchpress.com/servlet/linkout?suffix=refg8/ref8&dbid=16&doi=10.1139%2Fcjc-2015-0427&key=10.1039%2FC4EE01138D [26] XIAO M, HUANG F, HUANG W, et al.. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells[J]. Angew Chem. Int. Ed. Eng., 2014, 53(37):9898-9903. doi: 10.1002/anie.201405334 [27] YAN K, LONG M, ZHANG T, et al.. Hybrid halide perovskite solar cell precursors:colloidal chemistry and coordination engineering behind device processing for high efficiency[J]. J. Am. Chem. Soc., 2015, 137(13):4460-4468. doi: 10.1021/jacs.5b00321 [28] ZHANG W, SALIBA M, MOORE D T, et al.. Ultrasmooth organic-inorganic perovskite thin-film formation and crystallization for efficient planar heterojunction solar cells[J]. Nature Communications, 2015, 6:6142. doi: 10.1038/ncomms7142 [29] LEYDEN M R, ONO L K, RAGA S R, et al.. High performance perovskite solar cells by hybrid chemical vapor deposition[J]. J. Mater. Chem. A, 2014, 2(44):18742-18745. doi: 10.1039/C4TA04385E [30] STRANKS S D, NAYAK P K, ZHANG W, et al. Formation of thin films of organic-inorganic perovskites for high-efficiency solar cells[J]. Angew Chem. Int. Ed. Eng., 2015, 54(11):3240-3248. doi: 10.1002/anie.201410214 [31] SONG T B, CHEN Q, ZHOU H, et al.. Perovskite solar cells:film formation and properties[J]. J. Mater. Chem. A, 2015, 3(17):9032-9050. doi: 10.1039/C4TA05246C [32] CHEN Q, ZHOU H, HONG Z, et al.. Planar heterojunction perovskite solar cells via vapor-assisted solution process[J]. J. American Chemical Society, 2014, 136(2):622-625. http://www.ncbi.nlm.nih.gov/pubmed/24359486 [33] LUO P, LIU Z, XIA W, et al.. Uniform, stable, and efficient planar-heterojunction perovskite solar cells by facile low-pressure chemical vapor deposition under fully open-air conditions[J]. ACS Appl. Mater. Interfaces, 2015, 7(4):2708-2714. doi: 10.1021/am5077588 [34] DU T, WANG N, CHEN H, et al. Comparative study of vapor-and solution-crystallized perovskite for planar heterojunction solar cells[J]. ACS Appl. Mater. Interfaces, 2015, 7(5):3382-3388. doi: 10.1021/am508495r [35] ZHU W, YU T, LI F, et al.. A facile, solvent vapor-fumigation-induced, self-repair recrystallization of CH3NH3PbI3 films for high-performance perovskite solar cells[J]. Nanoscale, 2015, 7(12):5427-5434. doi: 10.1039/C5NR00225G [36] YOKOYAMA T, CAO D H, STOUMPOS C C, et al.. Overcoming short-circuit in lead-free CH3NH3SnI3 perovskite solar cells via kinetically controlled gas-solid reaction film fabrication process[J]. J. Phys. Chem. Lett., 2016, 7(5):776-782. doi: 10.1021/acs.jpclett.6b00118 [37] HAO F, STOUMPOS CC, LIU Z, et al.. Controllable perovskite crystallization at a gas-solid interface for hole conductor-free solar cells with steady power conversion efficiency over 10%[J]. J. American Chemical Society, 2014, 136(46):16411-16419. doi: 10.1021/ja509245x [38] XU J, YIN J, XIAO L, et al.. Bromide regulated film formation of CH3NH3PbI3 in low-pressure vapor-assisted deposition for efficient planar-heterojunction perovskite solar cells[J]. Solar Energy Materials and Solar Cells, 2016, 157:1026-1037. doi: 10.1016/j.solmat.2016.08.027 [39] CHEN J, XU J, XIAO L, et al.. Mixed-organic-cation (FA)x(MA)1-xPbI3 planar perovskite solar cells with 16.48% efficiency via a low-pressure vapor-assisted solution process[J]. ACS Applied Materials & Interfaces, 2017, 9(3):2449-2458. http://europepmc.org/abstract/MED/28054480 [40] LI Y, COOPER J K, BUONSANTI R, et al. Fabrication of planar heterojunction perovskite solar cells by controlled low-pressure vapor annealing[J]. J. Phys. Chem. Lett., 2015, 6(3):493-499. doi: 10.1021/jz502720a [41] WANG B, CHEN T. Exceptionally stable CH3NH3PbI3 Films in moderate humid environmental condition[J]. Adv. Sci.(Weinh), 2016, 3(2):1500262. doi: 10.1002/advs.201500262 [42] LEYDEN M R, LEE M V, RAGA S R, et al.. Large formamidinium lead trihalide perovskite solar cells using chemical vapor deposition with high reproducibility and tunable chlorine concentrations[J]. J. Mater. Chem. A, 2015, 3(31):16097-16103. doi: 10.1039/C5TA03577E [43] LUO P, LIU Z, XIA W, et al.. A simple in situ tubular chemical vapor deposition processing of large-scale efficient perovskite solar cells and the research on their novel roll-over phenomenon in J-V curves[J]. J. Mater. Chem. A, 2015, 3(23):12443-12451. doi: 10.1039/C5TA02306H [44] GOUDA L, GOTTESMAN R, TIROSH S, et al.. Vapor and healing treatment for CH3NH3PbI(3-x)Clx films toward large-area perovskite solar cells[J]. Nanoscale, 2016, 8(12):6386-6392. doi: 10.1039/C5NR08658B [45] 谢世伟, 肖啸, 谭建军, 等.基于石墨烯基电极染料敏化太阳能电池的研究进展[J].中国光学, 2014, 7(1):47-56. http://www.chineseoptics.net.cn/CN/abstract/abstract9095.shtmlXIE SH W, XIAO X, TAN J J, et al.. Recent progress in dye-sensitized solar cells using graphene-based electrodes[J]. Chinese Optics, 2014, 7(1):47-56. http://www.chineseoptics.net.cn/CN/abstract/abstract9095.shtml [46] ZHOU Z, XU J, XIAO L, et al. Efficient planar perovskite solar cells prepared via a low-pressure vapor-assisted solution process with fullerene/TiO2 as an electron collection bilayer[J]. RSC Adv., 2016, 6(82):78585-78594. doi: 10.1039/C6RA14372E -





 下载:
下载: